Chemistry - Online Test
Q1. For macromolecules to form, one more of the following criteria are essential
Answer : Option B
Explaination / Solution:
Macromolecule is made by joining smaller molecules(monomers)by covalent bond formation.
Q2. [Ar] 3d104s24p3 is the electronic configuration of
Answer : Option C
Explaination / Solution:
Arsenic is a group 15 element and its atomic no. is 33.
Q3. In a closed system, Which of the following take place ?
Answer : Option A
Explaination / Solution:
In a closed system, there is no flow of matter from system to surrounding or vice versa.
For example, a certain quantity of fluid bounded within a closed cylinder constitutes a closed system.
Q4. Metal which is found in free state is
Answer : Option B
Explaination / Solution:
Gold is least reactive metal.
Q5. Cathode rays or cathode ray particles are
Answer : Option D
Explaination / Solution:
Cathode rays - In 1897, British physicist J. J. Thomson showed the rays were composed of a previously unknown negatively charged particle, which was later named the electron.
Q6. The effect of undesirable changes in the environment causing harm to living beings is termed as:
Answer : Option B
Explaination / Solution:
Environmental pollution is the harmful effect of undesirable changes caused by substances of chemical, biological or human origin.
Q7. 4d transition elements series are from
Answer : Option D
Explaination / Solution:
4d series arises from Y ( 4d15s2 )
Q8.
Give IUPAC names of the following compound:
Answer : Option A
Explaination / Solution:
The IUPAC of the above compound will be more clear if we open up the structure:
The structure of above compounds is:
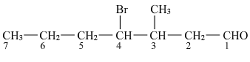
Hence the IUPAC name of above compound is 4-Bromo-3-methylheptanal.
Q9. Which of the following represents chelating ligand?
Answer : Option C
Explaination / Solution:


Q10. Hydrogen is the first element in the periodic table and has resemblance to alkali metals as well as with halogens. However, its placement in the periodic table has been a subject of discussion in the past. Currently hydrogen is placed in which group?
Answer : Option A
Explaination / Solution:
H properties matches with alkali metal as well as halogens so it is placed separately
