UNIT 8: Ionic Equilibrium - Online Test
Ag2C2O4
↔ 2Ag+
+ C2O42-
[ Ag+ ] = 2.24 × 10-4
mol L-1
[ C2 O42-
] = { 2.24×10-4 }/2 = mol L-1
= 1.12×10-4 mol L-1
Ksp = [Ag+]2[C2O42-]
= (2.24×10-4 mol-4 L-1
)2 (1.12×10-4 mol L-1)
= 5.619×10-12mol3
L-3
Following solutions were prepared by mixing different volumes of NaOH of
HCl different concentrations.
i. 60 mL (M/10) HCl + 40mL (M/10) NaOH
ii. 55 mL (M/10) HCl + 45 mL (M/10) NaOH
iii. 75 mL (M/5) HCl + 25mL (M/5) NaOH
iv. 100 mL (M/10) HCl + 100 mL (M/10) NaOH
iii) 75 ml M/5 HCl + 25ml M/5
NaOH
No of moles of HCl = 0.2×75×10-3
= 15 × 10-3
No of moles of NaOH = 0.2 × 25 ×
10-3 = 5 × 10-3
No of moles of HCl after mixing =
15 × 10-3 - 5 × 10-3
= 10 × 10-3
concentration
of HCl = No of moles of HCl / Vol in litre
= 10 ×10−3 / 100 ×10−3 = 0.1M
for (iii) solution, pH of 0.1M
HCl = -log10(0.1)
= 1.
BaSO4
↔ Ba2+
+SO42-
Ksp
=(s) (s)
Ksp =(s)2
= ( 2.42×10-3 g L-1
)2
= ( 2.42×10-3 g L-1
/ 233g mol-1 )
= ( 0.01038×10-3 )
2
= (1.038×10-5 )2
= 1.077×10-10
= 1.08×10-10 mol2
L-2
Ca(OH)2 ↔ Ca2+
+ 2OH-
Given that pH = 9
pOH = 14-9 = 5
[pOH = -log10 [OH- ]]
∴[OH- ] = 10- pOH
[OH- ]=10-5
M
Ksp =[Ca2+
][OH- ]2
= (10-5 /2 )×(10-5 )2
=0.5 ×10-15
H2O + H2O ↔ H3O+
+ OH-
acid 1 + base 1 ↔ acid 2 +
base 2
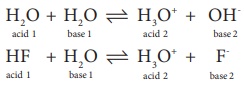
HF + H2O ↔ H3O+
+ F-
acid 1 + base 1 ↔ acid 2 +
base 2
Conjugate
bases are OH- and F- respectively
Basic
buffer is the solution which has weak base and its salt
NH4OH(200ml) + HCl(100ml) →NH4Cl(Salt) + H2O + NH4OH (100ml
weak base)
BF3 → elctron deficient → Lewis
acid
PF3 → electron rich → lewis
base
CF4 → neutral → neither
lewis acid nor base
SiF4- → neutral → neither
lewis acid nor base
BF3 → elctron deficient → Lewis
acid
PF3 → electron rich → lewis
base
CO → having
lone pair of electron → lewis base
F- → unshared pair of electron → lewis base
HCOONa Basic in nature. + H ⋅OH ↔ NaOH strong base + H-COOH weak acid
C6H5NH3Cl-
+ H ⋅ OH ↔ H3O+Acidic +C6H5 -NH2
+ Cl-
KCN basic + H− OH ↔ KOH
strong base + HCN weak acid
basic, acidic, basic is correct.
C5H5N + H-OH ↔ C5H5 NH + OH-
(α2C) / (1-α ) =Kb
α2C =∼ Kb
α = √ [ Kb
/ C ] = √[ 1.7 ×10-9 / 0.1 ]
= √1.7 × 10-4
= √1.7 × 10−4 × 100
Percentage of dissociation = 1.3 ×10-2 = 0.013 %
