UNIT 9: Solutions - Online Test
molality = number of moles of solute / weight of solvent (in
kg)
= (1.8/180) / 0.25 = 0.01/0.25 = 0.04M
Stomach acid, a dilute solution of HCl can be neutralised by
reaction with Aluminium hydroxide
Al (OH)3 + 3HCl (aq) → AlCl3 + 3 H2O
How many millilitres of 0.1 M Al(OH)3 solution are needed to
neutralise 21 mL of 0.1 M HCl ?
M1 × V1 = M2 × V2 [∵ 0.1 M
Al(OH)3 gives 3 × 0.1 = 0.3 M OH– ions]
0.3 × V1 = 0.1 × 21
V1 = 01x21 / 0.3 = 7ml
PN2= 0.76 atm
KH = 7.6 × 104
x = ?
PN2 = KH . x
0.76 = 7.6 × 104 × x
so x = 0.76 / [7.6 x104] = 1x10-5
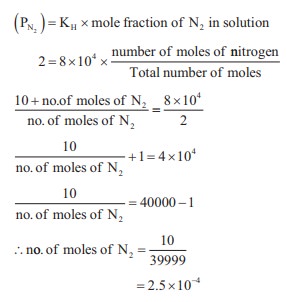
KH = 8 × 104
(xN2)in air = 0.5
Total pressure = 4 atm
pressure Partial pressure of nitrogen = mole fraction ×
total pressure = 0.5 × 4 = 2
for an ideal solution,
ΔSmix ≠ 0 ; Hence ΔGmix ≠ 0
∴ incorrect
is ΔGmix = 0
Ptotal = P1 + P2
= P1x1 + P2x2 (x1 + x2 = 1)
= P1 (1 – x2) + P2x2
(x1 = 1 – x2)
= P1 – P1x2 + P2x2
= P1 – x2 (P1 – P2)
π = CRT
π = n/V . RT
π V = nRT
