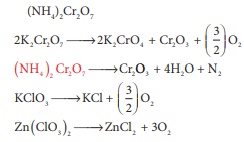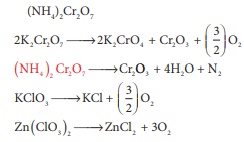Unit IV: Transition and Inner Transition Elements - Online Test
Q1. Sc( Z=21) is a transition element but Zinc (z=30) is not because
Answer : Option B
Explaination / Solution:
No Explaination.
Q2. Which of the following d block element has half filled penultimate d sub shell as well as half filled valence sub shell?
Answer : Option A
Explaination / Solution:
Cr ⇒ [Ar] 3d5 4s1
Q3. Among the transition metals
of 3d series, the one that has highest negative ( M2+ /M) standard electrode potential is
Answer : Option A
Explaination / Solution:
No Explaination.
Q4. Which one of the following
ions has the same number of unpaired electrons as present in V3+?
Answer : Option C
Explaination / Solution:
No Explaination.
Q5. The magnetic moment of Mn2+
ion is
Answer : Option A
Explaination / Solution:
Mn2+ => 3d5
contains 5 unpaired electrons
n=5 ; … = √n(n+2) BM
= √[5(5+2)] = √35 = BM
Q6. Which of the following compounds is colourless?
Answer : Option B
Explaination / Solution:
Ti4+ contains no
unpaired electrons in d orbital, hence no d-d transition.
Q7. the catalytic behaviour of transition metals and their compounds is ascribed mainly due to
Answer : Option C
Explaination / Solution:
No Explaination.
Q8. The correct order of increasing oxidizing power in the series
Answer : Option A
Explaination / Solution:
VO2+
{+5} < Cr2O72− {+6} < MnO4– {+7}
greater the oxidation state, higher is the xidizing power
Q9. The alloy of copper that contain Zinc is
Answer : Option D
Explaination / Solution:
Brass contains 70% Cu + 30% Zn
Q10. Which of the following does not give oxygen on heating?
Answer : Option B
Explaination / Solution: