UNIT 9: Electro Chemistry - Online Test
1F = 96500 C = 1 mole of e− = 6.023 ×1023
e−
∴ 9650 C = [ 6.22 ×1023 / 96500 ] × 9650 = 6.022×1022
= 6.022×1022
Consider the following half cell reactions:
Mn2+ + 2e− → Mn Eº = -1.18V
Mn2+ → Mn3+
+ e-
Eº = -1.51V
The E for the reaction 3Mn2+ → Mn+2Mn3+ , and the possibility of the
forward reaction are respectively.
Mn2+ + 2e− → Mn (Eºred) = -1.18V
2[Mn2+ → Mn3+
+ e- ](Eºox) = −1.51V
3Mn2+ → Mn + 2Mn3+ Eºcell?
Eºcell = ( Eºox )+ ( Eºred )
= −1.51 −1.18 and non spontaneous
= −2.69V
Since Eo is –ve ∆G is +ve and the given forward cell reaction is non
– spontaneous.
The button cell used in watches function as follows
Anodic
oxidation: (Reverse the given reaction)
( Eoox ) = 0.76V cathodic reduction
∴ Ecell = ( Eoox )+ ( Eredo )
= 0.76 + 0.34 = 1.1V
= 1.1V
Λ = κ/M ×10−3 mol−1 m3
= [( 5.76 × 10−3 S cm−1 ×10−3 ) / (0. 5)]
mol−1m3
= [(5.76 × 10−3 × 10−3 ×106 ) / (0.5)] S cm−1mol−1 cm3 .
= 11.52 S cm2 mol−1
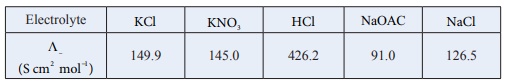
( Λº )HoAC = ( Λº)HCl + ( Λº)NaOAC - ( Λº)NaCl
= (426.2 + 91) −(126.5)
= 390.7
1F = 96500 C = charge of 1 mole
of e- =
charge of 6.022 ×1023 e−
7MnO-4 + 5e− → Mn2+ + 4H2O
5 moles of electrons i.e., 5F
charge is required.
m=ZIt
(41min 40sec = 2500 seconds)
Z = m/ (n × 96500) = 40 / (2 ×
96500)
m = Zit
= ( 40 × 3.86 × 2500 ) / (2 × 96500)
= 2g
m=ZIt
(mass of 1 mole of Cl2
gas = 71)
t = m/ ZI (∴mass of 0.1mole of Cl2 gas = 7.1 g mol−1 )
= 7.1 / [71/(2 ×96500) × 3 ]
(2 Cl- → Cl2 +2e- )
= (2 × 96500 × 7.1) / (71× 3)
= 6433.33sec
= 107.2 min
Q =It
= 1A×60S
96500 C charge ≡ 6.022 ×1023
electrons
60 C charge ≡ [ 6.022 ×1023
/ 96500
] × 60
= 3.744 ×1020 electrons
