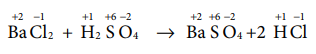UNIT 1: Basic Concepts of Chemistry and Chemical Calculations - Online Test
CH4(g) + 2O2(g) → CO2
(g)+ 2 H2O (l)
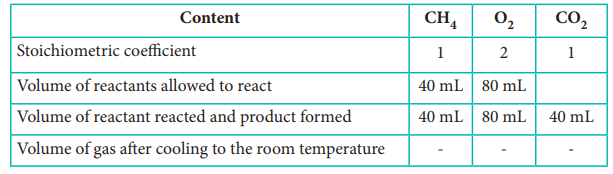
Since the
product was cooled to room temperature, water exists mostly as liquid. Hence,
option (a) is correct
Assertion Two mole of glucose contains 12.044 × 1023
molecules of glucose
Reason : Total number of entities present in one
mole of any substance is equal to 6.02 × 1022
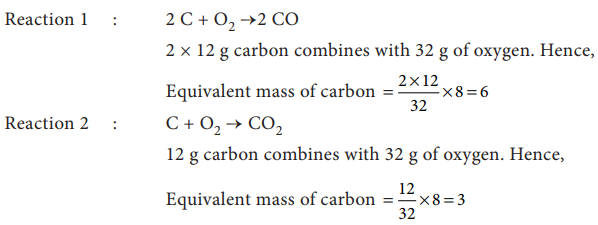
Let the
trivalent metal be M3+
Equivalent
mass = mass of the metal / valance factor
9 g eq-1
= mass of the metal / 3 eq
Mass of the
metal = 27 g
Oxide formed
M2O3 ;
Mass of the
oxide = (2 x 27) + (3 x 16)
= 102 g
Weight of the water drop =0.018 g
No. of moles of water in the drop=Mass of water /
molar mass
= 0.018 / 18
= 10-3 mole
No of water molecules present in 1 mole of water=
6.022 x 1023
No. water molecules in one drop of water (10-3
mole)= 6.022 x 1023x 10-3
=6.022 x
1020
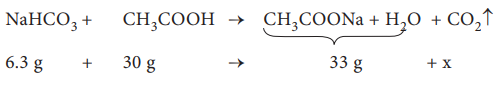
The amount of CO2 released, x = 3.3 g
No. of moles of CO2 released = 3.3 / 44
= 0.075 mol
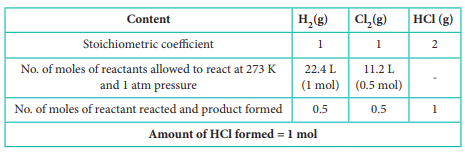
H2(g) + Cl2(g) → 2 HCl (g)