UNIT 11: Hydroxy Compounds and Ethers - Online Test
Q1. An
alcohol (x) gives blue colour in Victormeyer’s test and 3.7g of X when treated
with metallic sodium liberates 560 mL of hydrogen at 273 K and 1 atm pressure
what will be the possible structure of X?
Answer : Option A
Explaination / Solution:
2 R - OH + 2Na→ 2 RONa + H2↑2 moles of alcohol gives 1 mole of H2
which occupies 22.4L at 273K and 1 atm
∴ number of moles of alcohol = ( 2
moles of R - OH / 22.4 L of H2 ) × 560 mL
= 0.05 moles
∴ no. of moles = mass / molar mass
⇒ molar mass = 3.7/0.05 = 74 g mol−1
General
formula for R - OH Cn H2n+1 - OH
∴ n(12) + (2n+1) (1) + 16+1=74
14n = 74 – 18
14n = 56
∴ n = 56/14 = 4
The 2 alcohol which contains 4
carbon is CH2 CH(OH)CH2CH3
Q2. Which of the following compounds on reaction with methyl magnesium bromide will give tertiary alcohol.
Answer : Option C
Explaination / Solution:
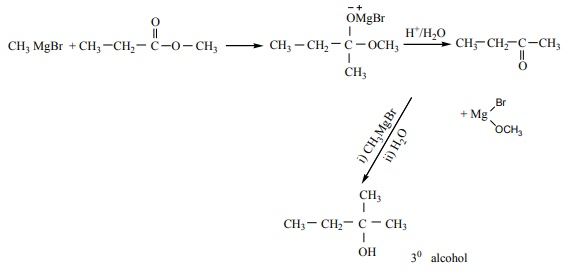

Q3. 

Answer : Option A
Explaination / Solution:
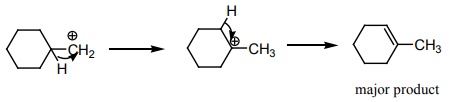
Hydro
boration – Anti markownikoft product i.e., CH3 - CH2 - CH
– CH2 – CH2 - OH
Q4. In
the reaction sequence, Ethene ----HOCl→A ---X→ ethan -1, 2 - diol . A and
X respectively are
Answer : Option C
Explaination / Solution:
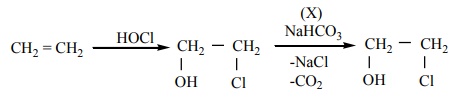
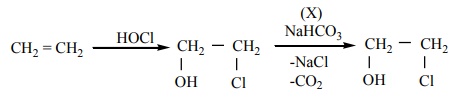
Q5. Which one of the following is the strongest acid
Answer : Option C
Explaination / Solution:
No Explaination.
Q6. 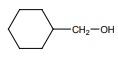 on treatment with Con H2 SO4 , predominately gives
on treatment with Con H2 SO4 , predominately gives
 on treatment with Con H2 SO4 , predominately gives
on treatment with Con H2 SO4 , predominately gives
Answer : Option B
Explaination / Solution:

Q7. Carbolic acid is
Answer : Option A
Explaination / Solution:
No Explaination.
Q8. Which one of the following will react with phenol to give salicyladehyde after hydrolysis.
Answer : Option C
Explaination / Solution:
Riemer
– Tiemann reaction 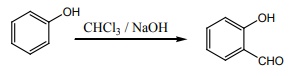

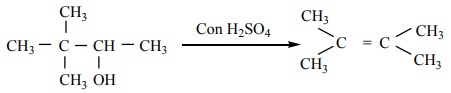
Q9. (CH3
)3 - C - CH(OH) CH3 -----Con H2 SO4→ X (major product)
Answer : Option B
Explaination / Solution:

Q10. The
correct IUPAC name of the compound, 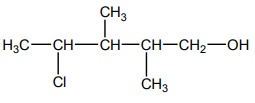

Answer : Option A
Explaination / Solution:
No Explaination.
