Chemistry - Online Test
Q1. Oxidation state of Iron and
the charge on the ligand NO in [ Fe ( H2O)5 NO] SO4 are
Answer : Option D
Explaination / Solution:
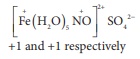
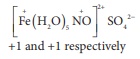
Q2.
Assertion : Acetamide on reaction with KOH and bromine gives acetic acid
Answer : Option D
Explaination / Solution:
both are wrong
Q3. Solid CO2 is an
example of
Answer : Option C
Explaination / Solution:
lattice points are occupied
by CO2 molecules
Q4. Glucose
---(HCN)→ Product ---(hydrolysis)→ Product ---(HI + Heat)→ A, the compound A is
Answer : Option A
Explaination / Solution:
No Explaination.
Q5. For a first order reaction
A → product with initial concentration x mol L−1 , has a half life period
of 2.5 hours . For the same reaction with initial concentration (x/2) mol L−1 the half life is
Answer : Option D
Explaination / Solution:
For a first order reaction
t1/2 = 0.693/k
t1/2 does not depend on the initial concentration and
it remains constant (whatever may be the initial concentration)
t1/2 = 2.5 hrs
Q6. Aspirin is a/an
Answer : Option A
Explaination / Solution:
No Explaination.
Q7. pH
of a saturated solution of Ca(OH)2 is 9. The Solubility product ( Ksp )of
Ca(OH)2
Answer : Option A
Explaination / Solution:
Ca(OH)2 ↔ Ca2+
+ 2OH-
Given that pH = 9
pOH = 14-9 = 5
[pOH = -log10 [OH- ]]
∴[OH- ] = 10- pOH
[OH- ]=10-5
M
Ksp =[Ca2+
][OH- ]2
= (10-5 /2 )×(10-5 )2
=0.5 ×10-15
Q8. The metal oxide which cannot be reduced to metal by carbon is
Answer : Option B
Explaination / Solution:
No Explaination.
Q9. The
molar conductivity of a 0.5 mol dm-3 solution of AgNO3
with electrolytic conductivity of 5.76 ×10−3 S cm−1 at 298 K is
Answer : Option B
Explaination / Solution:
Λ = κ/M ×10−3 mol−1 m3
= [( 5.76 × 10−3 S cm−1 ×10−3 ) / (0. 5)]
mol−1m3
= [(5.76 × 10−3 × 10−3 ×106 ) / (0.5)] S cm−1mol−1 cm3 .
= 11.52 S cm2 mol−1
Q10. Which of the following metals has the largest abundance in the earth’s crust?
Answer : Option A
Explaination / Solution:
No Explaination.
