Unit IV: Transition and Inner Transition Elements - Online Test
Q1. In acid medium, potassium permanganate oxidizes oxalic acid to
Answer : Option B
Explaination / Solution:
5(COO)22-
+ 2MnO 4 + 16H+ → 2Mn2+ + 10CO2 ↑ + 8H2O
Q2. Which of the following statements is not true?
Answer : Option B
Explaination / Solution:
No Explaination.
Q3. Permanganate ion changes to ________ in acidic medium
Answer : Option B
Explaination / Solution:
MnO4-
+ 8H+ + 5e- → Mn2+ + 4H2O
Q4. A white crystalline salt
(A) react with dilute HCl to liberate a suffocating gas (B) and also forms a
yellow precipitate . The gas (B) turns potassium dichromate acidified with dil
H2SO4 to a green coloured solution(C). A,B and C are
respectively
Answer : Option A
Explaination / Solution:
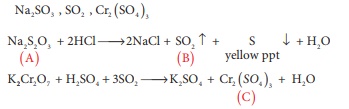
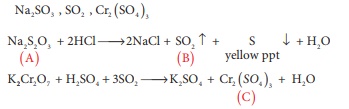
Q5. MnO4-
react with Br- in alkaline PH to give
Answer : Option A
Explaination / Solution:
2MnO4-
+ Br- + H2O → 2OH- + 2MnO2 + BrO3-
Q6. How many moles of I2
are liberated when 1 mole of potassium dichromate react with potassium iodide?
Answer : Option C
Explaination / Solution:
K2Cr2O7
+ 6KI + 7H2SO4 → 4 K2SO4 + Cr (SO4)3
+ 7H2O + 3I2
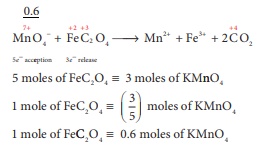
Q7. The number of moles of
acidified KMnO4 required to oxidize 1 mole of ferrous oxalate(FeC2O4)
is
Answer : Option C
Explaination / Solution:

Q8. When a brown compound of Mn
(A) ids treated with HCl , it gives a gas (B) . The gas (B) taken in excess
reacts with NH3 to give an explosive compound (C). The compound A, B
and C are
Answer : Option A
Explaination / Solution:
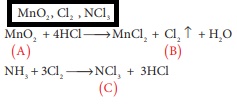
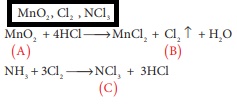
Q9. Which one of the following statements related to lanthanons is incorrect?
Answer : Option C
Explaination / Solution:
As we move from La to Lu , their metallic behaviour because almost similar to that of aluminium.
Q10. Which of the following lanthanoid ions is diamagnetic?
Answer : Option B
Explaination / Solution:
Yb2+ - 4f14
–no unpaired electrons – diamagnetic
