Unit II: p-Block Elements I - Online Test
Q1. Which of these is not a monomer for a high molecular mass silicone polymer?
Answer : Option A
Explaination / Solution:
No Explaination.
Q2. Which of the following is not sp2 hybridised?
Answer : Option D
Explaination / Solution:
dry ice – solid CO2 in which carbon
is in sp hybridized state
Q3. The geometry at which carbon atom in diamond are bonded to each other is
Answer : Option A
Explaination / Solution:
No Explaination.
Q4. Which of the following statements is not correct?
Answer : Option D
Explaination / Solution:
No Explaination.
Q5. AlF3 is soluble
in HF only in the presence of KF. It is due to the formation of
Answer : Option B
Explaination / Solution:
AlF3 + 3KF → K3[AlF6]
Q6. Match items in column - I
with the items of column – II and assign the correct code.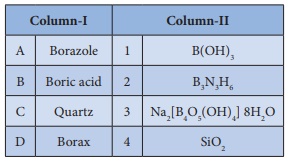

Answer : Option A
Explaination / Solution:
No Explaination.
Q7. Duralumin is an alloy of
Answer : Option D
Explaination / Solution:
Al-95% , Cu-4% , Mn-0.5% , Mn-0.5%
Q8. Thermodynamically the most stable form of carbon is
Answer : Option B
Explaination / Solution:
No Explaination.
Q9. The compound that is used in nuclear reactors as protective shields and control rods is
Answer : Option A
Explaination / Solution:
No Explaination.
Q10. The stability of +1 oxidation state increases in the sequence
Answer : Option A
Explaination / Solution:
stability of +1 oxidation state decreases down the group due to inert pair effect
