UNIT 8: Physical and Chemical Equilibrium - Online Test
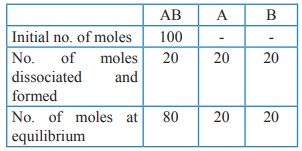
Total no. of moles at equilibrium = 80 + 20 + 20 = 120

For reaction given in option (a), (b) & (c) Δng = 0
For option (d) Δng = 2 – 1 = 1
∴ KP
= KC (RT)
If x is the fraction of PCl5 dissociated at
equilibrium in the reaction
PCl5 ⇌ PCl3
+ Cl2
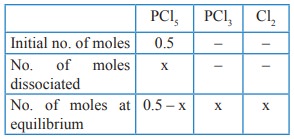
Total no. of moles at equilibrium = 0.5–x + x + x
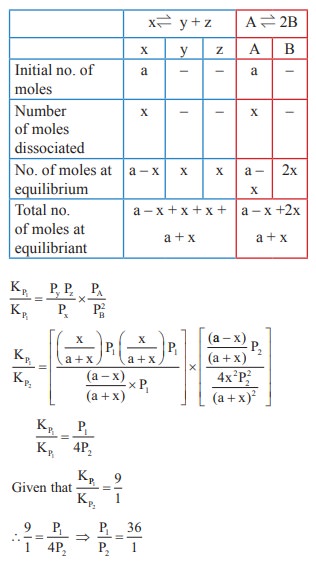
The values of K P1 and KP2 for the reactions
X ⇌ Y + Z
A ⇌ 2B are in
the ratio 9 : 1 if degree of dissociation and initial concentration of X and A
be equal then total pressure at equilibrium P1, and P2
are in the ratio
In the reaction,
Fe (OH)3 (s) ⇌ Fe3+(aq)
+ 3OH–(aq),
if the concentration of OH– ions is decreased by
¼ times, then the equilibrium concentration of Fe3+ will
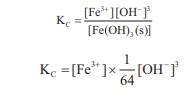
Consider the reaction where KP = 0.5 at a
particular temperature
PCl5(g) ⇌ PCl3
(g) + Cl2 (g)
if the three gases are mixed in a container so that the
partial pressure of each gas is initially 1 atm, then which one of the
following is true
KP = 0.5
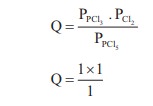
Q > KP ∴ Reverse
reaction is favoured ; i.e. more PCl5 will be produced. option (c)
V = 1L
H2 + I2 ⇌ 2HI
[H2]initial = [I2]initial
= a
[H2]eq = [I2]eq
= (a – x)
and [HI]eq = 2x
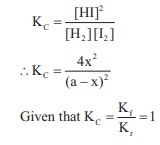
∴ 4x2
= (a – x)2
4x2 = a2 + x2 – 2ax
3x2 + 2ax – a2 = 0
x = –a & x = a/3
degree of dessociation = a/3× 100
= 33.33 %
Kf = 2.5 × 102
KC = 50
Kr = ?
Kc = Kf/Kr
50 = 2.5 x 102/ Kr
Kr = 5
correct statement : Physical processes occurs at the same
rate at equilibrium
∴ option
(c) is incorrect statement