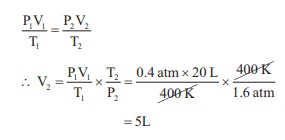UNIT 8: Physical and Chemical Equilibrium - Online Test
Match the equilibria with the corresponding conditions,
i) Liquid ⇌ Vapour
ii) Solid ⇌ Liquid
iii) Solid ⇌ Vapour
iv) Solute (s) ⇌ Solute
(Solution)
1. melting point
2. Saturated solution
3. Boiling point
4. Sublimation point
5. Unsaturated solution
A + B ⇌ C
KC= [C] / [A] [B]
if [A] and [B] are doubled, [C] increases 4 times to
maintain KC as constant.
∴
equilibrium constant will remain the same – option (d)
[Co(H2O)6]2+ (aq) (pink) +
4Cl– (aq) ⇌ [CoCl4]2–
(aq) (blue)+ 6 H2O (l)
on cooling, reverse reaction predominates and the solution
is pink in colour.
∴ decrease
in temperature, favours the reverse reaction ie reverse reaction is exothermic
and for the forward reaction is endothermic (ΔH > 0)
The equilibrium constants of the following reactions are :
N2 + 3H2 ⇌ 2NH3 ; K1
N2 + O2 ⇌ 2NO ; K2
H2 + ½O2 ⇌ H2O ; K3
The equilibrium constant (K) for the reaction ;
2NH3 + 5/2 O2 ↔k↔ 2NO+3H2O+3H2O,
will be
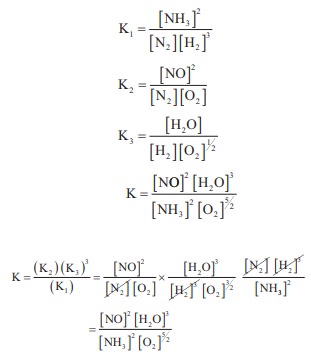
A 20 litre container at 400 K contains CO2 (g) at
pressure 0.4 atm and an excess of SrO (neglect the volume of solid SrO). The
volume of the container is now decreased by moving the movable piston fitted in
the container. The maximum volume of the container, when pressure of CO2
attains its maximum value will be :
Given that : SrCO3 (S) ⇌ SrO (S) + CO2(g)
Given that KP = 1.6 atm
V1 = 20 L
V2 = ?
T1 = 400 K
T2 = 400 K
KP = PCO2
∴ PCO2=
1.6 atm
P1 = 0.4 atm. P2 = 1.6 atm