UNIT 6: Gaseous State - Online Test
Q1. The
units of Vander Waals constants 'b' and 'a' respectively
Answer : Option C
Explaination / Solution:
an2/V2 = atm
= atm L2/mol2 = L2
mol-2 atm
nb = L
b= L/mol = L mol-1
Q2. Assertion
: Critical temperature of CO2 is 304K, it can be liquefied above
304K.
Reason
: For a given mass of gas, volume is to directly proportional to pressure at constant
temperature
Answer : Option D
Explaination / Solution:
Correct
Statement: Critical temperature
of CO2 is 304 K. It means that CO2 cannot be liquefied
above 304 K, whatever the pressure may applied.
Pressure is inversely proportional to volume
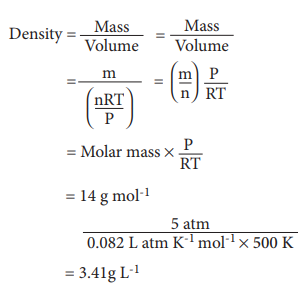
Q3. What
is the density of N2 gas at 227°C and 5.00 atm pressure? (R = 0.082
L atm K–1 mol–1)
Answer : Option C
Explaination / Solution:
Q4. Which
of the following diagrams correctly describes the behaviour of a fixed mass of
an ideal gas ? (T is measured in K)
Answer : Option C
Explaination / Solution:
For a fixed mass of an ideal gas V α T P α 1/V
and PV = Constant
Q5. 25g
of each of the following gases are taken at 27°C and 600 mm Hg pressure. Which
of these will have the least volume ?
Answer : Option D
Explaination / Solution:
At a given temperature and pressure
Volume α no. of moles
Volume α Mass / Molar mass
Volume α 28 / Molar mass
i.e. if molar mass is more, volume is less. Hence
HI has the least volume
