UNIT 6: Gaseous State - Online Test
Q1. Use
of hot air balloon in sports at meteorological observation is an application of
Answer : Option A
Explaination / Solution:
No Explaination.
Q2.
The gas which can be most easily liquefied is
The table indicates the value of van der Waals
constant ‘a’ in (dm3)2atm. mol-2
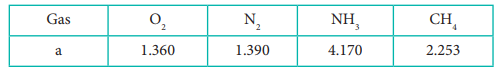
Answer : Option C
Explaination / Solution:
Higher
the value of 'a', greater the intermolecular force of attraction, easier the liquefaction.
Q3. Consider
the following statements
i. Atmospheric pressure is less at the top of a
mountain than at sea level
ii. Gases are much more compressible than solids or
liquids
iii. When the atmospheric pressure increases the
height of the mercury column rises Select the correct statement
Answer : Option D
Explaination / Solution:
No Explaination.
Q4. Compressibility
factor for CO2 at 400 K and 71.0 bar is 0.8697. The molar volume of
CO2 under these conditions is
Answer : Option C
Explaination / Solution:
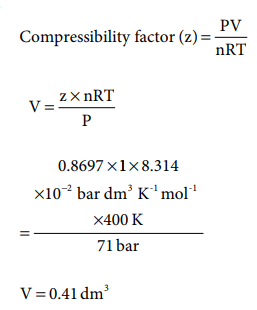
Q5. If
temperature and volume of an ideal gas is increased to twice its values, the
initial pressure P becomes
Answer : Option C
Explaination / Solution:
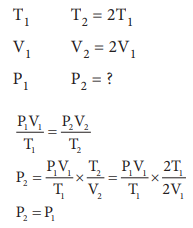
Q6. At
identical temperature and pressure, the rate of diffusion of hydrogen gas is
3√3 times that of a hydrocarbon having molecular formula CnH2n–2.
What is the value of n ?
Answer : Option B
Explaination / Solution:
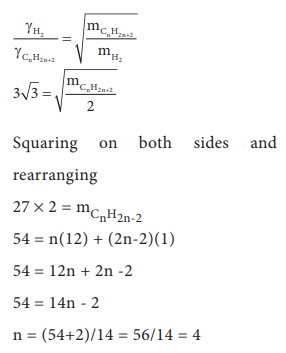
Q7. Equal
moles of hydrogen and oxygen gases are placed in a container, with a pin-hole
through which both can escape what fraction of oxygen escapes in the time
required for one-half of the hydrogen to escape.
Answer : Option C
Explaination / Solution:
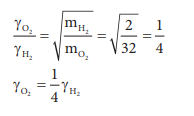
The fraction of oxygen that escapes in the time
required for one half of the hydrogen to escape is 1/8
Q8. The
variation of volume V, with temperature T, keeping pressure constant is called
the coefficient of thermal expansion ie α = 1/V (∂V/∂T)P. For an
ideal gas α is equal to
Answer : Option B
Explaination / Solution:
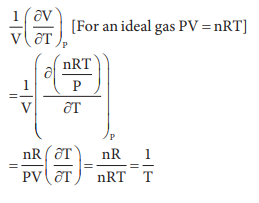
Q9. Four
gases P, Q, R and S have almost same values of 'b' but their 'a' values (a, b
are Vander Waals Constants) are in the order Q < R < S < P. At a
particular temperature, among the four gases the most easily liquefiable one is
Answer : Option A
Explaination / Solution:
Greater
the 'a' value, easier the liquefaction
Q10. Maximum
deviation from ideal gas is expected from
Answer : Option B
Explaination / Solution:
No Explaination.
