Chemistry - Online Test
Q1. Which of the following is an analgesic?
Answer : Option C
Explaination / Solution:
No Explaination.
Q2. Concentration
of the Ag+ ions in a saturated solution of Ag2 C2O4
is 2.24 ×10-4mol L-1
solubility product of Ag2 C2O4 is
Answer : Option D
Explaination / Solution:
Ag2C2O4
↔ 2Ag+
+ C2O42-
[ Ag+ ] = 2.24 × 10-4
mol L-1
[ C2 O42-
] = { 2.24×10-4 }/2 = mol L-1
= 1.12×10-4 mol L-1
Ksp = [Ag+]2[C2O42-]
= (2.24×10-4 mol-4 L-1
)2 (1.12×10-4 mol L-1)
= 5.619×10-12mol3
L-3
Q3. Bauxite has the composition
Answer : Option B
Explaination / Solution:
No Explaination.
Q4. The number of electrons that have a total charge of 9650 coulombs is
Answer : Option C
Explaination / Solution:
1F = 96500 C = 1 mole of e− = 6.023 ×1023
e−
∴ 9650 C = [ 6.22 ×1023 / 96500 ] × 9650 = 6.022×1022
= 6.022×1022
Q5. An aqueous solution of borax is
Answer : Option C
Explaination / Solution:
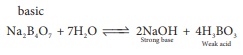
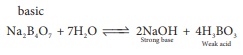
Q6. Which of the following is incorrect for physisorption?
Answer : Option B
Explaination / Solution:
The incorrect statement is option
(b)
Physisorption is an exothermic
process. Hence increase in temperature decreases the physisorption.
Q7. Which is true regarding nitrogen?
Answer : Option D
Explaination / Solution:
No Explaination.
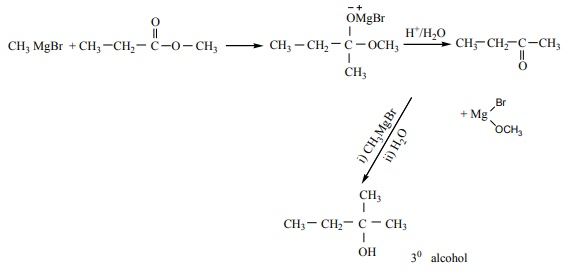
Q8. Which of the following compounds on reaction with methyl magnesium bromide will give tertiary alcohol.
Answer : Option C
Explaination / Solution:

Q9. Which of the following d block element has half filled penultimate d sub shell as well as half filled valence sub shell?
Answer : Option A
Explaination / Solution:
Cr ⇒ [Ar] 3d5 4s1
Q10. The formation of cyanohydrin from acetone is an example of
Answer : Option D
Explaination / Solution:
No Explaination.
