UNIT IX: Atomic and Nuclear Physics - Online Test
Q1. The mass of a 73Li nucleus
is 0.042 u less than the sum of the masses of all its nucleons. The binding
energy per nucleon of 73Li nucleus is nearly
Answer : Option B
Explaination / Solution:
Δm
= 0.042 u
Energy
of 1u=931 MeV
B.E
= 0.042 × 931
=
39.102 MeV
B.E
/ A = 39.102 / 7 = 5.6 MeV
Q2. Mp
denotes the mass of the proton and Mn denotes mass of a neutron. A
given nucleus of binding energy B, contains Z protons and N neutrons. The mass
M(N,Z) of the nucleus is given by(where c is the speed of light)
Answer : Option C
Explaination / Solution:
B = Δ mc2
B/c2 = Δm = NMn + ZMp − M(N,Z)
Q3. A radioactive nucleus (initial mass number A and atomic number Z) emits 2α and 2 positrons. The ratio of number of neutrons to that of proton in the final nucleus will be
Answer : Option B
Explaination / Solution:
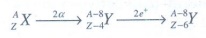
In
finial nucleus,
Number
of neutons:
NA
= A – 8 – (Z – 6) = A – Z – 2
Number
of protons:
Np
= Z – 6
Nn
/ Np = [ A – Z – 2 ] / [Z – 6]
Q4. The half-life period of a radioactive element A is same as the mean life time of another radioactive element B. Initially both have the same number of atoms. Then
Answer : Option C
Explaination / Solution:
(T1/2)A
= τB
0.693
/ λA = 1/ λB
λA
= 0.693 λB
λA < λB
B
will decay faster rate than A
Q5. A system consists of N0 nucleus at
t=0. The number of nuclei remaining after half of a half-life (that is, at time
t = 1/2 T1/2)
Answer : Option B
Explaination / Solution:
N
= (1/2)n (N0)
For
this case, n = (1/2)
So,
N = N0/√2
