UNIT IX: Atomic and Nuclear Physics - Online Test
Q1. Suppose an alpha particle accelerated by a potential of V volt is allowed to collide with a nucleus whose atomic number is Z, then the distance of closest approach of alpha particle to the nucleus is
Answer : Option A
Explaination / Solution:
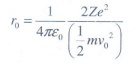
When
alpha particle is accelerated at the potential V;
K.E
= 2eV
Q2. In a hydrogen atom, the electron revolving in the fourth orbit, has angular momentum equal to
Answer : Option D
Explaination / Solution:
Angular
momentum L = n h
For
n = 4, L = 4h = 4h /2 π
=2h/ π
Q3. Atomic number of H-like atom with ionization potential 122.4 V for n = 1 is
Answer : Option C
Explaination / Solution:
The
ionization potential is
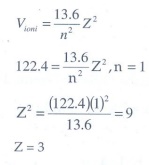
Q4. The ratio between the first three orbits of hydrogen atom is
Answer : Option C
Explaination / Solution:
The
radius of the nth orbit is rn
= n2r0
rn ∝ n2
r1:r2:r3 = 1:4:9
Q5. The charge of cathode rays is
Answer : Option B
Explaination / Solution:
Cathode ray is a beam of electrons
Q6. In J.J. Thomson e/m experiment, a beam of electron is replaced by that of muons (particle with same charge as that of electrons but mass 208 times that of electrons). No deflection condition is achieved only if
Answer : Option C
Explaination / Solution:
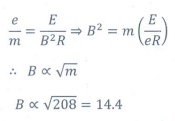
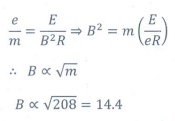
Q7. The ratio of the wavelengths for the transition
from n =2 to n = 1 in Li++, He+ and H is
Answer : Option D
Explaination / Solution:
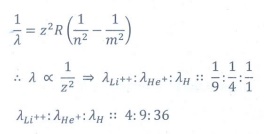
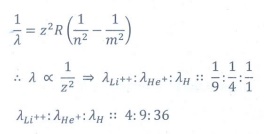
Q8. The electric potential between a proton and an
electron is given by V=V0ln ( r/r0 ) , where r0
is a constant. Assume that Bohr atom model is applicable to potential, then
variation of radius of nth orbit rn with the principal quantum
number n is
Answer : Option B
Explaination / Solution:
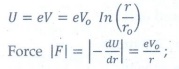
It
provide the centripetal force.
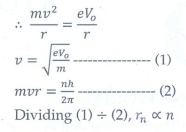
Q9. If the nuclear radius of 27Al is 3.6 fermi,
the approximate nuclear radius of 64Cu is
Answer : Option C
Explaination / Solution:
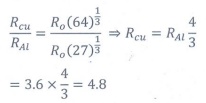
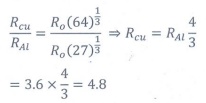
Q10. The nucleus is approximately spherical in shape. Then the surface area of nucleus having mass number A varies as
Answer : Option A
Explaination / Solution:
Surface
area = 4πR2
R=R0A1/3
Surface
area = 4π(R0A1/3)2
area
∝ A2/3
