UNIT 9: Electro Chemistry - Online Test
Q1.
Consider the change in oxidation state of Bromine corresponding to
different emf values as shown in the diagram below:
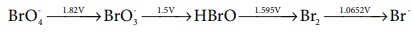
Then the species undergoing disproportionation is
Answer : Option D
Explaination / Solution:
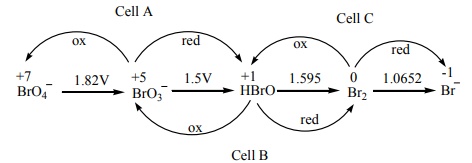
( Ecell )A =-1.82+1.5=-0.32V
( Ecell )B =-1.5+1.595=+0.095V
( Ecell )c =-1.595+1.0652=-0.529V
The species undergoing
disproportionation is HBrO
Q2.
For the cell reaction
2Fe3+ (aq) + 2l−(aq) → 2Fe2+ (aq) + l2 (aq)
Answer : Option A
Explaination / Solution:
No Explaination.
Q3. A certain current liberated 0.504gm of hydrogen in 2 hours. How many grams of copper can be liberated by the same current flowing for the same time through copper sulphate solution
Answer : Option B
Explaination / Solution:
No Explaination.
Q4. A gas X at 1 atm is bubbled through a solution containing a mixture of 1MY- and 1MZ- at 25°C . If the reduction potential of Z>Y>X, then
Answer : Option A
Explaination / Solution:
No Explaination.
Q5.
Cell equation : A + 2B- → A2+
+2B;
Answer : Option A
Explaination / Solution:
No Explaination.
