CBSE 12TH CHEMISTRY - Online Test
Q1. Liver when chronically exposed to chloroform gets damaged because
Answer : Option A
Explaination / Solution:
CHCl3 oxidises to form COCl2 which is a poison.
Q2. Which among the following is an instantaneous reaction?
Answer : Option A
Explaination / Solution:
Precipitation of AgCl is an example of instantaneous reaction.
Q3. In aerosol the dispersion medium is
Answer : Option C
Explaination / Solution:
Dispersion medium is gas.
Q4. The carbohydrate which cannot be hydrolysed in human digestive system is.
Answer : Option D
Explaination / Solution:
Cellulose which cannot be hydrolysed in human digestive system
Q5.
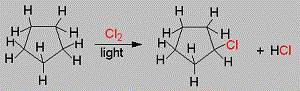
A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. The hydrocarbon is
Answer : Option A
Explaination / Solution:
Cyclopentane is nearly inert chemically, they react with halogens in the presence of light through the substitution of one hydrogen atoms. Since the cyclic structure confers a high degree of symmetry on the molecule, only one monochloro cyclopentane is possible.
Q6. Solution of hydrogen in palladium is an example of
Answer : Option A
Explaination / Solution:
Hydrogen (solute , gas ) and solvent is palladium (solid).
Q7. Benzene diazonium chloride reacts with phenol in which the phenol molecule at its para position is coupled with the diazonium salt to form p – hydroxyazobenzene
Answer : Option D
Explaination / Solution:
This normal N-N coupling.
Q8. Which among the following is a method of concentration of ore?
Answer : Option C
Explaination / Solution:
Leaching is method of concentration of ore. In which ore is digested with particular solvent to form a soluble complex.
Q9. Ammonia is used in detection of
Answer : Option D
Explaination / Solution:
Copper ions react with NH3 to form a blue color complex.
Q10. Which crystal system has axial angles equal to ?
Answer : Option C
Explaination / Solution:
Orthorhombic crystal system has axial angles
