CBSE 12TH CHEMISTRY - Online Test
Q1.
A solution of Ni()2 is electrolyzed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Answer : Option D
Explaination / Solution:


Q2. Anisole reacts with a mixture of concentrated sulphuric and nitric acids to yield a mixture of ortho and paranitroanisole


Q3. The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called
Answer : Option B
Explaination / Solution:
The minimum amount of energy required by the reacting molecules at the time of collisions in order to produce effective collisions is called threshold energy.
Q4. Which of the following methods is used for sol destruction?
Answer : Option A
Explaination / Solution:
Electrolyte makes the colloid destroy.
Q5. Nucleotides are joined together by
Answer : Option D
Explaination / Solution:
Nucleotides are together by phosphodiester linkage.
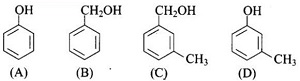
Q6.
Which of the following compounds is aromatic alcohol?
Answer : Option B
Explaination / Solution:
Compound (A) i.e., phenol and compound (D) i.e. a derivative of phenol cannot be considered as aromatic alcohol. As phenol is also known as, carbolic acid cannot be considered as aromatic alcohol.
Benzyl alcohol and substituted benzyl alcohol are aromatic alcohol.
Q7. Which has highest freezing point at 1 atm?
Answer : Option A
Explaination / Solution:
Sugar solution has i=1 so ΔTf minimum so Tf will be maximum.
Q8. In Pyridine, preferred site of nucleophilic substitution is one of the following positions
Answer : Option B
Explaination / Solution:
Due to resonance, this position is electron rich and steric hindrance will be least.
Q9. In the extraction of nickel by Mond’s process, the metal is obtained by
Answer : Option A
Explaination / Solution:
Ni is first reacted with CO to form Nickel tetracarbonyl which is volatile. From this complex Nickel is extracted by thermal decomposition.
Q10. The structure of ClF3 is
Answer : Option B
Explaination / Solution:
CN=0.5(V+M-C+A)
For ClF3 . CN= 5 so hyb is sp3d . Structure is trigonal bipyramidal.