CBSE 12TH CHEMISTRY - Online Test
Q1. How much charge is required for the reduction of 1 mol of to Cu?
Answer : Option C
Explaination / Solution:
For reduction of 1 mol of to Cu, 2 mol of electrons are required so total charge will be 2F.
Q2. Triiodomethane (Iodoform) is
Answer : Option C
Explaination / Solution:
The compound finds small scale use as a disinfectant.Around the beginning of the 20th century it was used in medicine as a healing and antiseptic dressing for wounds and sores, although this use is now superseded by superior anticeptics..
Q3. Which among the following is an example of first order reaction?
Answer : Option A
Explaination / Solution:
Decomposition of is 1st order reaction.
Q4. Which of the following metal solution cannot be prepared by Bredig’s arc method?
Answer : Option D
Explaination / Solution:
Potassium due to very high reactivity, canot be used here.
Q5. Adenosine is.
Answer : Option A
Explaination / Solution:
Adenosine is nucleoside.
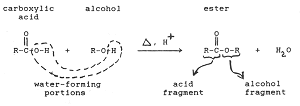
Q6. Reaction of an alcohol with organic acid is called the __________
Answer : Option C
Explaination / Solution:
Esterification is the reaction in which a Carboxylic acid combines with an alcohol in the presence of little concentrated sulphuric acid to form an ester. The esters so formed are pleasant smelling.
Q7. A solute is soluble in two immiscible liquids which are present in a mixture. The concentration of the solute in the upper layer will be
Answer : Option A
Explaination / Solution:
A solute distributes itself between two immisible liquids. Ratio of conc of solute in liquid1 and liquid 2 is constant.
Q8. When a 1° amine reacts with an alkyl sulfonyl chloride, the major organic product is __________.
Answer : Option D
Explaination / Solution:
Sulphonyl chloride reacts with primary amine to form sulphonamide.
RNH2+RI SO2Cl → RI SO2NHR + HCl
Q10. Which of the following metals cannot be extracted by smelting process?
Answer : Option A
Explaination / Solution:
Al cannot be extracted by smelting.

