CBSE 12TH CHEMISTRY - Online Test
Q1. The correct name of the compound [Cu(NH3)4](NO3)2 is
Answer : Option C
Explaination / Solution:
Tetraamminecopper(II)nitrate is [Cu(NH3)4](NO3)2
Q2. The disadvantage of using Penicillin is
Answer : Option A
Explaination / Solution:
Pencillin causes allergic reactions.
Q3. The resistance of a conductivity cell containing 0.001M KCl solution at 298 K is 1500 Ω. What is the cell constant if conductivity of 0.001M KCl solution at 298 K is 0.146 × 10–3 S .
Answer : Option D
Explaination / Solution:
Κ= G x cell constant and G= 1/R.
Q4. Toluene reacts with a halogen in the presence of iron (III) chloride giving ortho and para halo compounds. The reaction is
Answer : Option C
Explaination / Solution:
This is example of electrophilic aromatic substitution (EAS) reaction.Sincle Cl ion replaces hydrogen atom and here FeCl3 acts as halogen carriers.
Q5. Reaction which takes place in one step is known as
Answer : Option C
Explaination / Solution:
Elementary reaction are one step reactions.
Q6. Which adsorption takes place at low temperature?
Answer : Option D
Explaination / Solution:
Physical adsorption is favoured at low temperature because it involves only vanderwall interactions between adsorbate and adsorbent.
Q7. The sugar constituent of DNA is.
Answer : Option A
Explaination / Solution:
Sugar constituent of DNA is D-2-deoxy ribose.
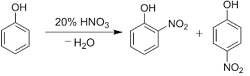
Q8. With dilute nitric acid at low temperature (298 K),phenol yields
Answer : Option D
Explaination / Solution:
Nitration of phenols: Phenols upon treatment with dilute nitric acid undergoes nitration at low temperature (298 K) to give a mixture of ortho and para nitrophenols. The mixture formed is further separated into ortho and para nitrophenols by steam distillation on the basis of their volatility. Due to intramolecular and intermolecular hydrogen bonding, ortho nitrophenols are lesser volatile in comparison to para nitrophenols which involves only intermolecular hydrogen bonding.
Q9. Which among the following is soluble in n-octane?
Answer : Option B
Explaination / Solution:
Like dissolves in like
Q10. When pentanal reacts with ethylamine under conditions of acid catalysis, the major organic product is __________.
Answer : Option C
Explaination / Solution:
Imines are typically prepared by the condensation of primary amines and aldehydes and less commonly ketones:
- RNH2 + R'C(O)R → RN=C(R')(R) + H2O
