CBSE 12TH CHEMISTRY - Online Test
Q1. Calculate the standard cell potentials of galvanic cell, and equilibrium constant of the reactions if the reaction is


Answer : Option D
Explaination / Solution:


Q2. The following compound is ,as per the IUPAC system


Answer : Option C
Explaination / Solution:
Longest chain contains double bond.
Q3. The role of catalyst in a chemical reaction is to change the
Answer : Option D
Explaination / Solution:
Role of catalyst is to decrease the activation energy of reaction.
Q4. The adsorbent used to adsorb the dye particles in the dying industry is
Answer : Option B
Explaination / Solution:
Alum is used in dying industries.
Q5. Which of the following is most soluble in water?
Answer : Option B
Explaination / Solution:
As they have polar interactions in water hence are soluble.
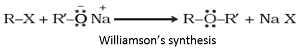
Q6. The reaction

is known as

is known as
Answer : Option A
Explaination / Solution:
Williamson’s synthesis: When an alkyl halide reacts with sodium alkoxide, ether is formed. This reaction is known as Williamson’s synthesis. The reaction generally follows SN2 mechanism for primary alcohols.
Q7. Which among the following is an example of liquid in solid?
Answer : Option A
Explaination / Solution:
Hydrated salts have water molecule of hydration as solute.
Q8. Heating a(n) __________ results in a Cope elimination.
Answer : Option D
Explaination / Solution:
Heating of Amine oxide result in cope elimination cis elimination.
Q9. Which of the following turns lead acetate paper black?
Answer : Option D
Explaination / Solution:
Pb2++S2−→PbS(black)
Q10. Nature of binding forces present in carbondioxide molecules in solid state are
Answer : Option D
Explaination / Solution:
O=C=O<−>−O−C+=O
