CBSE 12TH CHEMISTRY - Online Test
Q1. Chlordiazepoxide, meprobamate and equanil are
Answer : Option D
Explaination / Solution:
Theya re tranquillisers.
Q2. In which of the following complexes, the nickel metal is in the highest oxidation state?
Answer : Option C
Explaination / Solution:
In A Ni has +4 oxidation state.
Q3. Fuel cells are ________ that are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into_________.
Answer : Option A
Explaination / Solution:
Fuel Cells converts energy coming from the combustion of fuels directly to electrical energy.
Q4. In the reaction

Pent – 2 – ene. 2 – Bromopentane. Pent – 1 – ene, 2 – Bromopentane on heating with alcoholic KOH, forms two compounds: Pent – 1 – ene and Pent – 2 – ene., if one major and one product are formed , then

Pent – 2 – ene. 2 – Bromopentane. Pent – 1 – ene, 2 – Bromopentane on heating with alcoholic KOH, forms two compounds: Pent – 1 – ene and Pent – 2 – ene., if one major and one product are formed , then
Answer : Option B
Explaination / Solution:
The major alkene will ne more substituted that what zaitsev rule is.
Q5. A reaction whose order is different from the actual due to large excess concentration of one of the reactants is called
Answer : Option C
Explaination / Solution:
The concentration of the reactants used in excess remains unaltered during the course of the reaction.
Q6. Ammonia is adsorbed by
Answer : Option A
Explaination / Solution:
Charcoal act as an adsorbent.
Q7. Protein is generally a polypeptide with _______ amino acid residues
Answer : Option A
Explaination / Solution:
In protein more than 100 amino acids combine to form proteins
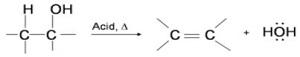
Q8. Alcohols undergo dehydration (removal of a molecule of water) to form alkenes on treating with
Answer : Option C
Explaination / Solution:
The dehydration reaction of alcohols to generate alkene proceeds by heating the alcohols in the presence of a strong acid, such as sulfuric or phosphoric acid, at high temperatures.
The required range of reaction temperature decreases with increasing substitution of the hydroxy-containing carbon:
- 1° alcohols: 170° - 180°C
- 2° alcohols: 100°– 140 °C
- 3° alcohols: 25°– 80°C
If the reaction is not sufficiently heated, the alcohols do not dehydrate to form alkenes, but react with one another to form ethers.
Q9. Vapour pressure of a liquid is constant at constant temperature because
Answer : Option B
Explaination / Solution:
Liquid and vapours are in equilibrium.
Q10. Which of the following is a tertiary amine?
Answer : Option D
Explaination / Solution:
Benadryl have tertiary amine.