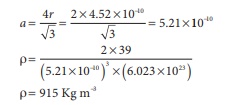Unit VI: Solid State - Online Test
Q1. The ionic radii of A+
and B− are 0.98 × 10−10 m and 1.81 × 10−10 m . the coordination number of each ion in AB is
Answer : Option C
Explaination / Solution:
rC+/ rA-
= 0.98 ×10−10 / 1.81 ×10−10 = 0.54
it is in the range of 0.
414 - 0.732 , hence the coordination number of each ion is 6
Q2. CsCl has bcc arrangement, its unit cell edge length is 400pm, its inter atomic distance is
Answer : Option D
Explaination / Solution:
Solution
√3 a = rCs+ +
2rCl- + rCs+
(√3/2) a = ( rCl-
+ rCs+)
(√3/2) x 400 = inter
ionic distance
Q3. A solid compound XY has NaCl structure. if the radius of the cation is 100pm , the radius of the anion will be
Answer : Option A
Explaination / Solution:
for an fcc structure rx+ / ry− = 0.414
given that rX+ = 100 pm
r y− = 100 pm
/ 0.414
Q4. The vacant space in bcc lattice unit cell is
Answer : Option C
Explaination / Solution:
packing efficiency = 68%
therefore empty space
percentage = (100-68) = 32%
Q5. The radius of an atom is 300pm, if it crystallizes in a face centered cubic lattice, the length oif the edge of the unit cell is
Answer : Option B
Explaination / Solution:
let edge length = a
√2a = 4r
a = [4 × 300] / √2
a = 600 ×1.414
a = 848.4 pm
Q6. The fraction of total volume occupied by the atoms in a simple cubic is
Answer : Option B
Explaination / Solution:
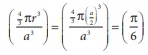
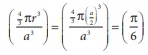
Q7. The yellow colour in NaCl crystal is due to
Answer : Option A
Explaination / Solution:
No Explaination.
Q8. if ‘a’ stands for the edge length of the cubic system ; sc , bcc, and fcc. Then the ratio of radii of spheres in these systems will be respectively.
Answer : Option C
Explaination / Solution:
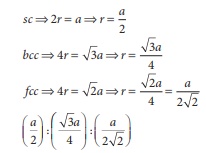
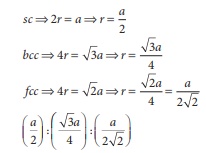
Q9. If ‘a’ is the length of the side of the cube, the distance between the body centered atom and one corner atom in the cube will be
Answer : Option D
Explaination / Solution:
if a is the length of the side, then the length of the leading
diagonal passing through the body centered atom is √3a
Required distance (√3/2) a
Q10. Potassium has a bcc structure with nearest neighbor distance 4.52 Aº . its atomic weight is 39. its density will be
Answer : Option A
Explaination / Solution:
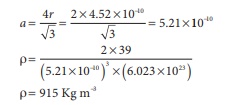
ρ = n × M / a3NA
for bcc
n = 2
M=39
nearest distance 2r =
4.52