CBSE 12TH CHEMISTRY - Online Test
Q1. Ag+ ion is isoelectronic with
Answer : Option B
Explaination / Solution:
Ag+ is isoelectronic with Cd2+
Q2. K3CoF6 is high spin complex. What is the hybrid state of Co atom in this complex?
Answer : Option B
Explaination / Solution:
CN is 6 so octahedral complex with hybridization sp3d2 .
Q3. Some of the detergents are non – biodegradable because
Answer : Option D
Explaination / Solution:
The branched carbon chain is difficult to degrade.
Q4. A strong base can abstract an α – hydrogen from
Answer : Option A
Explaination / Solution:
Because conjugate base that form will be stable in case of Ketone.
Q5. The highest electrical conductivity of the following aqueous solutions is of
Answer : Option C
Explaination / Solution:
Acidity increases on attaching electron withdrawing group because of stability of conjugate base.
Q6. A dibromo derivative of an alkane reacts with sodium metal to form an alicyclic hydrocarbon. The derivative is ______.
Answer : Option B
Explaination / Solution:
Intramolecular wurtz leading to cyclisaion.
Q7. The rate law for the reaction is given by rate= k[RCl]. The rate for this reaction
Answer : Option C
Explaination / Solution:
Since order of reaction with respect to RCl is one so if conc of Rcl is halfed the rate of reaction will also become half.
Q8. The process of separation of colloids by passing through semi permeable membrane is called
Answer : Option C
Explaination / Solution:
Dialysis we use, salt solution of approximately same concentration outside the artificial blood capillary, so that urea comes out of the blood, but other useful substances like NaCl , blood cell etc does not. Blood is a colloid.
Q9. Simple protein is.
Answer : Option B
Explaination / Solution:
Simple protein is Albumin.
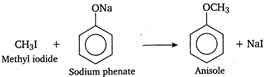
Q10. Anisole can be prepared by the action of methyl iodide on sodium phenate. The reaction is called
Answer : Option D
Explaination / Solution:
The
reaction of alkyi halide with sodium alkoxide to give ether (alkoxy
alkane), is known as Williamsons synthesis. In this reaction an ether
(anisole) is prepared by the action of alkyi halide (methyl iodide) on
sodium alkoxide (sodium phenate), so it is an example of Williamsons
synthesis.