CBSE 12TH CHEMISTRY - Online Test
Q1. Which of the following complexes does not obey the EAN rule?
Answer : Option C
Explaination / Solution:
23+12=35 EAN is not followed.
Q2. A patient suffering from sleeplessness was advised to take one of the following medicine in small dosages. Which is it?
Answer : Option A
Explaination / Solution:
Valium is used treat sleeplessness problem.
Q3. Resistance of 0.2 M solution of an electrolyte is 50 Ω. The specific conductance of the solution is 1.3 S . If resistance of the 0.4 M solution of the same electrolyte is 260 Ω, its molar conductivity is
Answer : Option C
Explaination / Solution:
κ= G × cell constant and G= 1/R.


Q4.
A hydrocarbon C5H10 does not react with chlorine in dark but gives a single monochloro compound
C5H9C l in bright sunlight. The hydrocarbon is
Answer : Option D
Explaination / Solution:
Alkanes undergoes halogenations in prrsence of light.
Q5. The half life periods of a reaction at initial concentration 0.1 mol/L and 0.5 mol/L are 200 s and 40 s respectively. The order of the reaction is
Answer : Option D
Explaination / Solution:
As initial concentration is increased half life is decreasing so order of reaction is 2.
Q6. Flocculation value is expressed in terms of
Answer : Option A
Explaination / Solution:
Fact
Q7. Which one is not the essential amino acid in the ones given below?
Answer : Option C
Explaination / Solution:
Leucine is not essential amino acid
Q8. Which of the following species can act as the strongest base?
Answer : Option B
Explaination / Solution:
Hydroxy group is more electron donating group than alkoxy because alkoxy have greater number of atoms than that hydroxyl group there electronic density of oxygen of alkoxy group spreads over the whole group and greater the number of atom less will be the electron donating ability.
Q9.
Give IUPAC name of the compound given below.

Answer : Option A
Explaination / Solution:
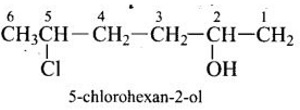
-OH is given preference over -Cl so numbering is done so that –OH gets the lowest number.
Q10. A solution showing a large positive deviation from ideal behaviour has
Answer : Option C
Explaination / Solution:
A solution showing a large positive deviation from ideal behaviour shows lower boiling point than both the components.
