CBSE 12TH CHEMISTRY - Online Test
Q1. The colloidal solution of two immiscible liquids is called
Answer : Option A
Explaination / Solution:
Dispersed phase is liquid, dispersion medium is liquid.
Q2. D – ribose and 2 – deoxy – D – ribose are
Answer : Option C
Explaination / Solution:
They are pentose sugar
Q3.
IUPAC name of the following compound is

Answer : Option B
Explaination / Solution:
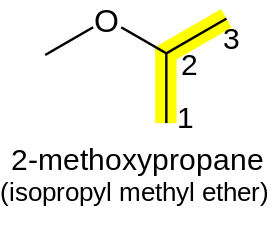
If the oxygen is not attached to the end of the main alkane chain, then the whole shorter alkyl-plus-ether group is treated as a side-chain and prefixed with its bonding position on the main chain. Thus CH3OCH(CH3)2 is 2-methoxypropane.
Q4. Molarity is preffered over molarity in handling solutions in chemistry laboratory because
Answer : Option A
Explaination / Solution:
Molarity depends upon Volume of solution which changes with Temperature.
Q5. Which of the following respond to the isocyanide test?
Answer : Option B
Explaination / Solution:
Only primary amines undergo carbylamines reaction (isocyanide test)
Q6. Which of the following is used as for manufacturing conveyor belts, gaskets and hoses.
Answer : Option D
Explaination / Solution:
Neoprene is used in the manufacturing of Conveyor belts and hoses.
Q7. Most metal oxides are
Answer : Option B
Explaination / Solution:
In general, metal oxides are basic and ionic in nature.
Q8. The process in which the concentrated ore is heated to a high temperature in the absence of air is known as
Answer : Option B
Explaination / Solution:
Carbonate ore is heated to a high temperature in the absence of air is calcination.
Q9. Which of the following pairs of ions have same paramagnetic moment?
Answer : Option C
Explaination / Solution:
Cu2+, Ti3+ will have same unpaired electrons and hence same paramagnetic moment.
Q10. IUPAC name of the following compound is


Answer : Option A
Explaination / Solution:
This is 1-methoxy-1-methylethane.
