CBSE 12TH CHEMISTRY - Online Test
Q1. ATP is
Answer : Option B
Explaination / Solution:
ATP is nucleotide.
Q2. Alkenes react with water in the presence of acid as catalyst to form alcohols.
Answer : Option A
Explaination / Solution:
The addition of water to an alkene in the presence of a catalytic amount of strong acid leads to the formation of alcohols (hydroxy‐alkanes).
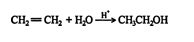
This reaction proceeds via a standard carbocation mechanism and follows the Markovnikov rule.
The mechanism for the addition of water to ethene follows.
1. The hydrogen ion is attracted to the π bond, which breaks to form a σ bond with one of the double‐ bonded carbons. The second carbon of the original double‐bonded carbons becomes a carbocation.
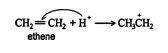
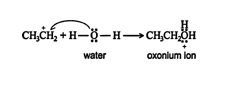
2.An acid‐base reaction occurs between the water molecule and the carbocation, forming an oxonium ion.
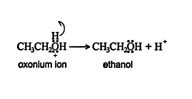
3. The oxonium ion stabilizes by losing a hydrogen ion, with the resulting formation of an alcohol.
Q3. Camphor is often used in molecular mass determination because
Answer : Option D
Explaination / Solution:
Camphor has very high cryoscopic constant.
Q4. Which of the following is a tertiary amine?
Answer : Option D
Explaination / Solution:
this is tertiary amine.
Q5. The furnace which gives the highest temperature is
Answer : Option D
Explaination / Solution:
Blast furnance maintains the highest temperature.
Q6. Which of the following is Paramagnetic?
Answer : Option D
Explaination / Solution:
NO is paramagnetic. Its unpaired electron is present in antibonding orbital.
Q7. The process by which impurity is introduced in semiconductors to enhance its conductivity is known as
Answer : Option D
Explaination / Solution:
Semiconductors are doped with impurities to enhance conductivity.
Q8. Which of the following statements is not true about low density polythene?
Answer : Option B
Explaination / Solution:
Low density polymer are poor conductor of electricity.
Q9. Which shows maximum magnetic moment among the bivalent ions of the first transition series?
Answer : Option B
Explaination / Solution:
Mn2+ has d5 configuration so maximum no. of un paired electrons.
Q10. A mixture of benzaldehyde and formaldehyde on heating with aqueous NaOH solution gives
Answer : Option C
Explaination / Solution:
They will undergo cannizaro reaction.
