CBSE 11TH CHEMISTRY - Online Test

Chemistry does not deal in explaining superconductivity.
*In superconducting materials the charecteristics of superconductivity appear when the temperature is lowered below a critical temperature.
*The onset of superconductivity is accompanied by abrupt changes in physical properties which are more related to phase transitions of the material .
These aspects of studies in properties of materials are better related to studies in the fields of Physics , eventhough principles involved in Chemistry & Physics go hand to hand.
In the following sequence of reactions, the alkene is converted to compound
The compound is --------- ?
The given sequence of conversion steps represent Ozonolysis of 2-Butene , which follows the following path ,
1 . Formation of an unstable intermediate / ozonide
2 . Cleavage of the intermediate / ozonide by to smaller molecules , giving out the compound , which is CH3 CHO ( Ethanal )
The reaction is well depicted as below ,
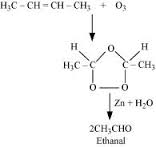 ,
,
How many protons, neutrons and electrons are in ?
Atomic number of iron (Fe) = 26
So number of protons = 26
Number of protons = number of electrons
But To gain 3+ charge it should loose 3 electron. So number of electrons present = 26-3 = 23
Now atomic mass = 56 (Given).
Number of neutrons = atomic mass - number of protons = 56-26 = 30
