CBSE 11TH CHEMISTRY - Online Test
Q1. Calculate the volume occupied by 8.8 g of CO2 at 31.1 and 1 bar pressure. R = 0.083 bar .
Answer : Option B
Explaination / Solution:
V=wRTMPV=8.8×0.083×304.144×1=5.05L
Q2. An oxidation number of +2 is found in all their compounds of one of the below given options
Answer : Option D
Explaination / Solution:
Alkaline earth metals have in common an outer s- electron shell which is full; that is, that is why orbital contains its full complement of two electrons, which these elements readily lose to form cations with charge +2, and an oxidation state (oxidation number) of +2.
Q3. For the reaction

of is combusted in 1000 g of O2. The yield of and the limiting agent in the reaction are

of is combusted in 1000 g of O2. The yield of and the limiting agent in the reaction are
Answer : Option C
Explaination / Solution:
moloes of given = 51.72 mol Moles of O2 = 31.25 mol. Since 2 mol of requires 13 mol of so 51.72 mol of require 336.18 mol of but we have is only 31.25 mol therefore is limiting reagent. Therefore 13 mol of gives 10 mol of so 31.25 mol will give 24.038 mol of water = 432 g of water.
Q4. The aqueous solution of sugar does not conduct electricity. However, when sodium chloride is added to water, it conducts electricity. How will you explain this statement on the basis of ionisation and how is it affected by concentration of sodium chloride?
Answer : Option A
Explaination / Solution:
NaCl ionises completely in water and increase in concentration of salt increases conductance.
Q5. A dibromo derivative of an alkane reacts with sodium metal to form an alicyclic hydrocarbon.
The derivative is ______.
Answer : Option D
Explaination / Solution:
1,4 - dibromobutane ( ie. a dibromoderivative of an alkane , butane) when reacted withl in ethereal solution produces alicyclic hydrocarbon, Cyclobutane as per following reaction.
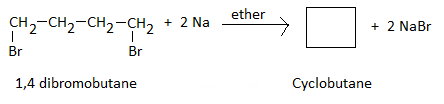
Therefore , the dibromo-derivative of alkane is identified as 1 , 4-dibromobutane.
Q6. The correct increasing order of radii of following species is ----?
Answer : Option B
Explaination / Solution:
It is because
(i) radius of anion ( anionic radius ) is greater , and
(ii) radius of cation ( cationic radius ) is lesser
than that of its parent neutral atom.
Q7. Lines in the hydrogen spectrum which appear in the ultraviolet region of the electromagnetic spectrum, then they are called as
Answer : Option A
Explaination / Solution:
The Lyman series is a hydrogen spectral series of transitions and resulting ultraviolet emission lines of the hydrogen atom as an electron goes from n ≥ 2 to n = 1 (where n is the principal quantum number), the lowest energy level of the electron.
Q8. Carbon dioxide and oxygen levels are maintained by:
Answer : Option D
Explaination / Solution:
Carbon dioxide and oxygen levels are maintained by Photosynthesis by plants and respiration by organisms.
Q9. Calculate the strength of 5 volume solution.
Answer : Option B
Explaination / Solution:
Volume strength = 11.2 x M
So M == 0.446
Strength (g/L ) = 0.446 x 34 = 15.17 g/L
Q10.
for the reaction . Calculate for the reaction, and predict whether the reaction may occur spontaneously.
Answer : Option C
Explaination / Solution:


