CBSE 11TH CHEMISTRY - Online Test
Q1. An oxidation number of +1 is found in all their compounds of one of the below given options
Answer : Option B
Explaination / Solution:
The alkali metals (Group 1) have 1 valence electron. Alkali metals looses this electron to achieve noble gas configuration, and so alkali metals have oxidation number +1.
Q2.
Given that Avagadro’s Number
= 6.02 x 1023 atoms/mol ,
the number of hydrogen atoms in 18g of water is.
Answer : Option B
Explaination / Solution:
Since,18g H2O
= 1mol water containing 6.02 x 1023 molecules of water (H2O )
& the number of H atoms in 1 molecule of hydrogen (H2) = 2
the number of H atoms in 6.02 x 1023 molecules
= (6.02 x 1023 x 2)
=1.204 x 1024.
Q3. Among the following compounds the one that is most reactive towards electrophilic nitration is
Answer : Option A
Explaination / Solution:
Methyl group is electron donating group, hence it increases the electron density in benzene ring thereby increasing the reactivity of the ring towards electrophilic substitution.
Q4. Calculate the enthalpy change on freezing of 1.0 mol of water at 10.0 to ice at -10.0. H = 6.03 kJ at 0.1
Answer : Option C
Explaination / Solution:


Q5. Right order of increasing metallic character is:
Answer : Option A
Explaination / Solution:
Elements of s block are more matallic than p block elements
Q6.
The electronic configuration belongs to
Answer : Option A
Explaination / Solution:
Boron is a chemical element with symbol B and atomic number 5. So electronic configuration of boron is
Q7. Which of these atmospheric pollutants is not released by car exhausts ?
Answer : Option D
Explaination / Solution:
Magnesium oxide is not released by car exhaust because Mg is not present in the fuels.
Q8. Metal hydrides are ionic, covalent or molecular in nature. Among LiH, NaH, KH, RbH, CsH, the correct order of increasing ionic character is
Answer : Option B
Explaination / Solution:
As the size of cation increases ionic character also increases.
Q9. The number of types of bonds between two carbon atoms in calcium carbide is
Answer : Option C
Explaination / Solution:
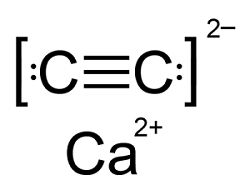
CaC2 has a combination of bonds. It is an ionic lattice that has Ca2+ cations and acetylide C22- anions. Within each C22-there is a triple bond between the 2C atoms, consisting of 1 sigma and 2 pi bonds.
Q10. To acquire noble gas configuration, alkaline earth metals lose
Answer : Option D
Explaination / Solution:
Alkaline Earth Metals loses 2 electron to attain noble gas configuration.
