CBSE 11TH CHEMISTRY - Online Test
Q1. Arrange the following alkyl halides in decreasing order of the rate of elimination reaction with alcoholic KOH
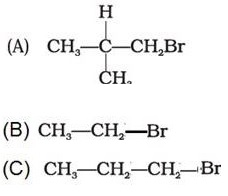

Answer : Option B
Explaination / Solution:
A more substituted alkene is preferred in accordance with the Saytzeff's rule.Hence the order.
Q2. One of the following has ns1 as its outermost electronic configuration
Answer : Option D
Explaination / Solution:
The elements of Group 1 (alkali metals) which have ns1 outermost electronic configuration belong to the s-Block Elements.
Q3.
Wave number of yellow radiations having wavelength of 5800 .
Answer : Option B
Explaination / Solution:
Wave number is defined as the reciprocal of wavelength.
where, = wavelength = 5800 Å =
So,
Q4. Sulphur dioxide levels can be reduced by using :
Answer : Option C
Explaination / Solution:
Sulphur dioxide levels can be reduced by using Low sulphur fuels so that less amount of SO2 is produced from them
Q5.
Why does H+ ion always get associated with other atoms or molecules?
Answer : Option D
Explaination / Solution:
Loss of an electron from hydrogen atom results in a nucleus of very small size as compared to other atoms or ions.
H+ ion has exceptionally small size, so it can not exist freely.
Q6. The structure of is
Answer : Option C
Explaination / Solution:
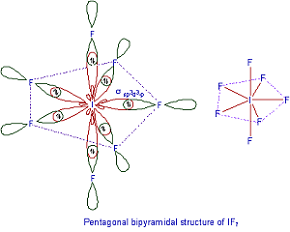
In IF7 out of 7 Flourine atoms 5 of them are placed on a plane in Pentagon shape. In remaining 2 flourines one is placed above the plane and other below the plane each at 90 degrees
Q7. Potassium ions are the most abundant cations. They:
Answer : Option C
Explaination / Solution:
Potassium ions are the most abundant cations. They participate in the oxidation of glucose to produce ATP
Q8. In which C-C bond of the inductive effect is expected to be the least?
Answer : Option C
Explaination / Solution:
As distance increases inductive effect also decreases.
Q9. 34.05 mL of phosphorus vapour weighs 0.0625 g at 546 and 0.1 bar pressure. What is the molar mass of phosphorus?
Answer : Option B
Explaination / Solution:
PV=(0.0625/M)RT where p=0.1bar T=819.15K and V= 34.05ml
Q10. In which of the following solvents is silver chloride most soluble?
Answer : Option A
Explaination / Solution:
AgCl is soluble in ammonia due to the formation of complex
