CBSE 11TH CHEMISTRY - Online Test
Q1. The molar mass of Al2O3 is
Answer : Option A
Explaination / Solution:
Molar mass
= [ 2 x ( atomic mass of Al ) , 3 x ( atomic mass of O ) ]u
=
= ( 54 + 48 ) u
= 102 u.
Q2. Which of the following will not show geometrical isomerism?
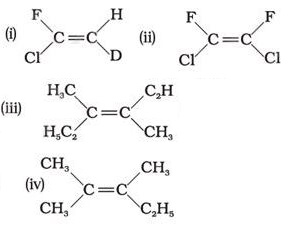

Answer : Option C
Explaination / Solution:
This is because geometrical isomerism is not possible if three groups are same.
Q3. A reaction, A + B → C + D + q is found to have a positive entropy change. The reaction will be
Answer : Option C
Explaination / Solution:
ΔG = ΔH -T ΔS
ΔS is positive and ΔH is negative as heat is liberated in the reaction.
so ΔG is negative hence reaction will be spontaneous at all temperature.
Q4. The outer electronic configuration of f - Block elements are:
Answer : Option C
Explaination / Solution:
The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) – Lu(Z = 71) and Actinoids, Th(Z = 90) – Lr (Z = 103) are characterised by the outer electronic configuration (n-2)f1-14 (n-1)d0-1ns2. The last electron added to each element is filled in f- orbital.
Q5. Television pictures result due to
Answer : Option B
Explaination / Solution:
Cathode rays (also called an electron beam or e-beam) are streams of electrons observed in vacuum tubes. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass behind of the positive electrode is observed to glow, due to electrons emitted from and traveling away from the cathode (the electrode connected to the negative terminal of the voltage supply).
Cathode ray tubes (CRTs) use a focused beam of electrons deflected by electric or magnetic fields to create the image in a television set
Q6. Which of these reactions in the atmosphere leads to acid rain ?
Answer : Option B
Explaination / Solution:
2SO2 + O2 + 2H2O ------> 2H2SO4 is the major reaction of acid rain. SO2 emitted from burning of fossil fuels containing S react with O2 and H2O to form the acid leading to acid rain.
Q7. In the process of obtaining pure de-mineralised water is passed initially through cation exchange, This makes the water
Answer : Option C
Explaination / Solution:
Cation exchange resin have exchangeable hydrogen ions which makes the water acidic.
Q8. The maximum number of hydrogen bonds that a molecule of water can have is
Answer : Option B
Explaination / Solution:
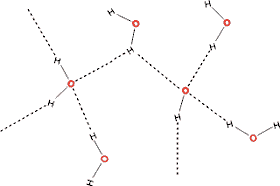
The two hydrogens of the water molecule can form hydrogen bonds with other oxygens in water, and the two lone pair of electrons on oxygen of the water molecule can attract other hydrogens in water. Hence, 4 possible hydrogen bonds.
Q9. The common salt or table salt is
Answer : Option C
Explaination / Solution:
Common salt is NaCl.
Q10. The organic compound given below is one of the four options given below. Choose the most appropriate one.


Answer : Option A
Explaination / Solution:
this is aliphatic heterocyclic compound.
