CBSE 11TH CHEMISTRY - Online Test
Metal hydrides (MHx) are the most technologically relevant class of hydrogen storage materials because they can be used in a range of applications including neutron moderation,electrochemical cycling, thermal storage, heat pumps, and purification/separation.
The metal hydrides such as intermetallic alloys and solid solutions have interstitial vacancies where atomic hydrogen is absorbed via an exothermic reaction; however, by endothermic path, the metal hydride desorbs the hydrogen reversibly at ambient to moderate temperatures.

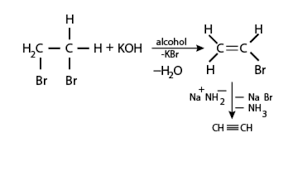
Elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either one or two steps .When the starting dihalide is a vicinal dihalide , alkyne is obtained by two successive elimination reactions.
Example :
Vicinal dihalide, on treatment with forms a monosubstituted alkene Vinyl bromidewhich when reacted with sodium amide , forms ethyne (ie. an alkyne ). The steps of conversion are ,
This is redox couple.
Where following reaction take place :
Zn2+ + 2e- Zn
