CBSE 11TH CHEMISTRY - Online Test
Q1. For dissolution of solids in liquids, at a given temperature, the constant is
Answer : Option D
Explaination / Solution:
This is considered at equilibrium process.
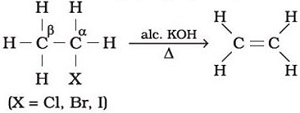
Q2.
One of the methods of preparing ethylene is given below.This is an example of _______ reaction
Answer : Option B
Explaination / Solution:
Dehydration of alcohols in the presence of alc. KOH to form an alkene is an example of elimination reaction.
Q3. Standard electrode potential of three metals X, Y and Z are –1.2 V, +0.5 V and –3.0 V respectively. The reducing power of these metals will be
Answer : Option A
Explaination / Solution:
X=-1.2V , Y=+0.5V , Z=-3.0V Therefore, Z>X>Y
Because, higher the reduction potential ,lesser the reducing power.
Q4. The alkali metals and their salts impart characteristic colour to an oxidizing flame because:
Answer : Option C
Explaination / Solution:
Alkali metals have one unpaired electron in their valence shell which gets excited by absorbing the energy from flame, excited states are very unstable and quickly move back to lower state ,at that time they emit the energy in the form of radiation in visible reason of electromagnetic spectrum and is abserved as characteristic colour.
Q5. Standard Molar Enthalpy of Formation is
Answer : Option B
Explaination / Solution:
The standard enthalpy change for the formation of one mole of a compound from its elements in their most stable states of aggregation is enthalpy of formation.
Q6. The third period (n = 3) begins at sodium with addition of first electron to
Answer : Option B
Explaination / Solution:
The electronic configuration of sodium is 1s2 2s2 2p6 3s1. The valence electron is in 3s orbital.
Q7.
Mg2+ is isoelectronic with
Answer : Option D
Explaination / Solution:
Isoelectronic species refers to the elements that have the same number of electrons.
Mg2+ is a 10 electron species .Its configuration is like that of Ne.
Thus it is isoelectronic with any element having 10e- or we can say 8e- in its valence shell.
Atomic number of Sodium (Na) is 11 after loosing one electron it became Na+ and have 10 electron.
Thus Mg2+ is isoelectronic with Na+.
Q8. A given compound always contains exactly the same proportion of elements by weight. This law is stated by
Answer : Option D
Explaination / Solution:
The observation was first made by French chemist Joseph Proust , based on certain experiments conducted between 1798 and 1804.
Proust made the above statement known as " Proust's law " or " Law of definite composition " or " Law of constant composition "
Q9. Inadequate pollution control equipments result in the :
Answer : Option C
Explaination / Solution:
Inadequate pollution control equipments release greater amount of carbon monoxide that increases the pollution even further.
Q10. The ionic hydrides are crystalline, non-volatile but on melting does one of the following:
Answer : Option C
Explaination / Solution:
They are formed when hydrogen molecule reacts with most s-block elements which are highly electropositive in nature. In solid state, the ionic hydrides are crystalline, non-conducting and non-volatile.
However, in liquid state it conducts electricity. Ionic hydrides on electrolysis liberate hydrogen gas at the anode.