CBSE 11TH CHEMISTRY - Online Test
Q1. Find energy of the photon which has wavelength of 0.50 Å. (Hint: h = Planck’s constant = 6.626 × J)
Answer : Option D
Explaination / Solution:
Energy (E) of a photon having wavelength (λ) is given by the expression,
where , h = Planck’s constant =
c = velocity of light in vacuum =
Wavelength
Substituting the values in the given expression of E:
Q2. Global warming will not result in:
Answer : Option C
Explaination / Solution:
Global warming will not increase the size of the hole in the ozone layer as temperature does not affect the depletion.
Q3. Consider the reactions (A)
Answer : Option B
Explaination / Solution:
is an oxidizing agent in 1st reaction and Reducing agent in 2nd reaction.
Q4. for the reaction at 298 K, = 400 kJ and = 0.2 kJ At what temperature will the reaction become spontaneous considering and to be constant over the temperature range.
Answer : Option B
Explaination / Solution:
ΔG= ΔH-TΔS At equilibrium ΔG=0 then T= ΔH/ΔS =2000K
Q5. The hybridization of central atom of a molecule would lead to
Answer : Option D
Explaination / Solution:
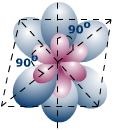
In sp3d2 Hybridization one s, three p and two d-orbitals get hybridized to form six sp3d2 hybrid orbitals which adopt octahedral arrangement as given in image below
Q6. Radium salts are used in
Answer : Option D
Explaination / Solution:
Ra salts are used in the treatment of Cancer.
Q7. Geometrical isomers and Optical isomers are:
Answer : Option C
Explaination / Solution:
They are stereoisomers as they differ in the orientation of atoms in space.
Q8.
Pay load is defined as the difference between the mass of displaced air and the mass of the balloon. Calculate the pay load when a balloon of radius 10 m, mass 100 kg is filled with helium at 1.66 bar at. (Density of air = 1.2 kg m-3 and R = 0.083 bar .
Answer : Option B
Explaination / Solution:
Volume of balloon,V=4/3(3.14) =4187 Mass of displaced air,m=V × δ =4187 x 1.2=5024.4 kg Temperature,T=300 K Moles of gas present,n=PV/RT=279.1 × 103 moles Mass of helium=279.1 ×103 x 4=116.4 kg Mass of filled balloon,m'=100+1116.4=1216.4 kg Payload=m-m'=3811.1 kg
Q9. Using the standard electrode potential, find out the pair between which redox reactions is not feasible.
Answer : Option D
Explaination / Solution:
As for this couple is negative so this couple is not possible.
Q10. What is the mass of 1 mole of O2 ?
Answer : Option B
Explaination / Solution:
Calculations:
Mass of 1mole of O2
= the mass of 6.022 x 1023 molecules of O2 ' ( as per Avogadro's law )
= molar mass of O2 in grams ( ie. gram molar mss )
= 32 .0 g
